
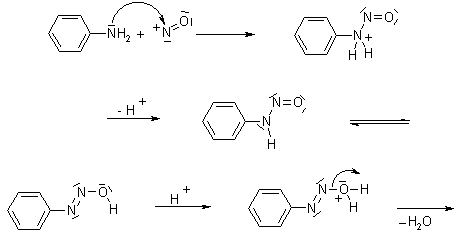
The nitrosation of primary aromatic amines with nitrous acid (generated in situ from sodium nitrite and a strong acid, such as hydrochloric acid, sulfuric acid, or HBF4) leads to diazonium salts, which can be isolated if the counterion is non-nucleophilic.
Diazonium salts are important intermediates for the preparation of halides (Sandmeyer Reaction, Schiemann Reaction), and azo compounds. Diazonium salts can react as pseudohalide-type electrophiles, and can therefore be used in specific protocols for the Heck Reaction or Suzuki Coupling.
The intermediates resulting from the diazotization of primary, aliphatic amines are unstable; they are rapidly converted into carbocations after loss of nitrogen, and yield products derived from substitution, elimination or rearrangement processes.
Mechanism


No comments:
Post a Comment